Aromatic L-amino Acid Decarboxylase (AADC) Deficiency
What is AADC Deficiency?
Aromatic l-amino acid decarboxylase (AADC) deficiency is a rare genetic disorder that affects the brain.1 It interferes with the way the cells in the nervous system talk to each other through a person’s neurotransmitters. In AADC deficiency, a genetic mutation leads to a decrease in the amount of neurotransmitters made by the body.1,2,3
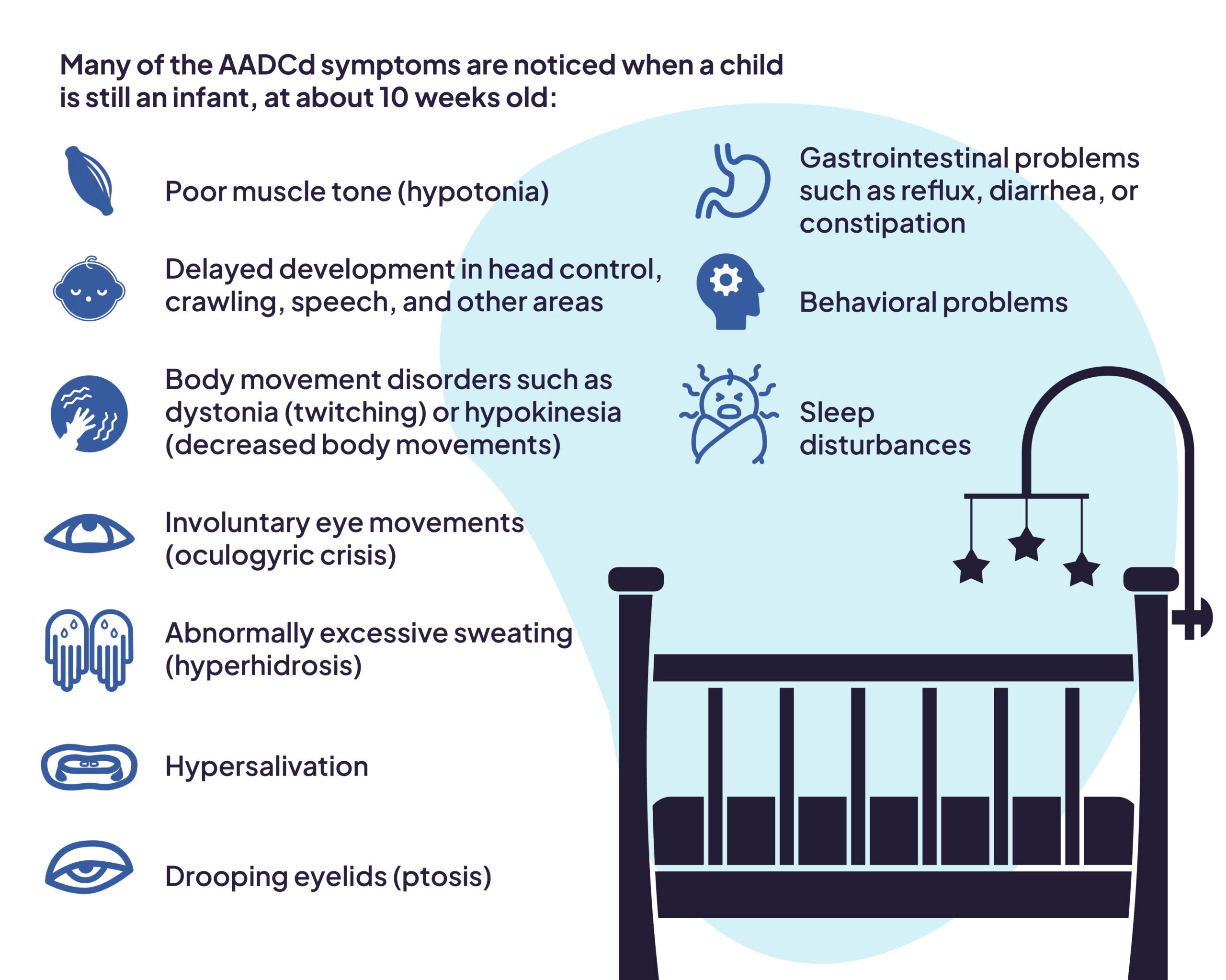
AADC deficiency manifests with several key characteristics and symptoms. Many of these symptoms are noticed when a child is still an infant, at about 10 weeks old:1,2,3,4
- Poor muscle tone (hypotonia)
- Delayed development in head control, crawling, speech, and other areas
- Body movement disorders such as dystonia (twitching) or hypokinesia (decreased body movements)
- Involuntary eye movements (oculogyric crisis)
- Abnormally excessive sweating (hyperhidrosis)
- Hypersalivation
- Drooping eyelids (ptosis)
- Gastrointestinal problems such as reflux, diarrhea, or constipation
- Behavioral problems
- Sleep disturbances
How Common is AADC Deficiency? Is it Treatable?
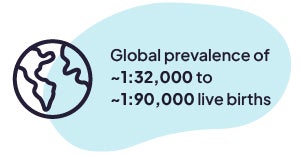
AADC deficiency is an extremely rare disease.1 Because its symptoms are similar to those of other diseases, it can be difficult and time-consuming to diagnose.1,3 At this time, there is no cure for AADC deficiency.1
Certain treatments can sometimes help improve a child’s symptoms.1 A pediatrician or general practitioner can advise on how to provide occupational or physical therapies which can improve quality of life. In addition, a doctor might refer AADC deficiency patients to a pediatric neurologist, a movement disorder specialist, a clinical geneticist or another specialist who can help identify relevant and helpful treatments.1
How Can You Stay Informed About AADC Deficiency?
There are multiple ways to become an AADC deficiency-informed parent, caregiver or health care provider.
If you are a parent or caregiver, please visit AboutAADC.com
If you are a healthcare professional, please visit AADCInsights
If you are interested in no-cost AADC deficiency testing, please refer your physician to the following website for details: https://aadcinsights.com/
[1] Wassenberg T, et al. Orphanet J Rare Dis. 2017;12(1):12.
[2] Himmelreich N, et al. Mol Genet Metab. 2019;127(1):12-22.
[3] Pearson T et al. J Inherit Metab Dis. 2020 Sep;43(5):1121-1130.
[4] Saberian S et al. Poster presented at ISPOR 2021, 30 November – 3 December, 2021
[5] PTC Therapeutics Data on File, Upstaza Summary of Product Characteristics (SmPC). July 2022.
Do you have questions?
Please reach out if you would like to speak with us.