Friedreich's Ataxia (FA)
What is Friedreich’s Ataxia?
Friedreich’s ataxia (FA) is a rare, physically debilitating, life-shortening, neuromuscular disorder that mainly affects the central nervous system and the heart.1 It is the most common hereditary ataxia (abnormal, uncoordinated movements) and is usually caused by a single genetic defect in the frataxin (FXN) gene that leads to reduced production of frataxin, a mitochondrial protein that is important for cellular metabolism and energy production.1,2 Decreased frataxin levels are associated with mitochondrial iron accumulation and increased oxidative stress, which can lead to cell death through ferroptosis.3,4,5
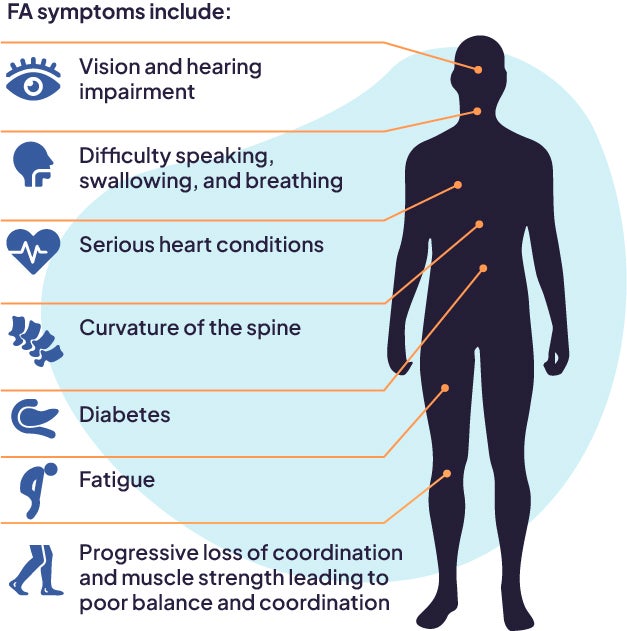
Symptoms include progressive loss of coordination and muscle strength leading to poor balance and coordination, difficulty speaking, swallowing, and breathing, curvature of the spine, serious heart conditions, diabetes, and hearing and vision impairment.6,7 The severity of symptoms and speed of progression varies between people and some symptoms may not be evident in all. Friedreich’s ataxia is usually diagnosed in childhood or adolescence.2,8
How is PTC Working to Treat Friedreich’s Ataxia?
PTC is developing a potential treatment for FA based on our Inflammation & Ferroptosis platform.


Briana L. Johnson Manager, Clinical Operations, PTCI am very passionate about helping to improve the quality of life of those living with FA. Through working with PTC’s Patient Engagement team, I had the opportunity to attend and support several advocacy events in the FA community. Getting to know this community of patients and families has been so fulfilling; they are my motivation.
Hear from patients and caregivers impacted by FA on our My VIBE podcast

Do you have questions?
Please reach out if you would like to speak with us.
[1] Lynch DR, Farmer JM, Balcer LJ, et al. Arch Neurol 2002;59(5):743–747.
[2] Campuzano V, Montermini L, Lutz Y, et al. Hum Mol Genet 1997;11(6):1771–1780.
[3] Campuzano V, et al. Hum Mol Genet. 1997;6:1771–1780.
[4] Cook A, Giunti P. Br Med Bull. 2017;124:19–30.
[5] Pandolfo M, Hausmann L. J Neurochem. 2013;126:142–146.
[6] Bürk K. Cerebellum Ataxias 2017;4:4.
[7] Cook A, Giunti P. Br Med Bull 2017;124(1):19–30.
[8] Delatycki MB, Williamson R, Forrest SM. J Med Genet 2000;37(1):1–8.
[9] Hinman A, et al. PLoS one. 2018;13:e0201369.
[10] PTC Therapeutics. EPI-743 Pre-Clinical Data Deck.
[11] Shrader WD, et al. Bioorg Med Chem Lett. 2011;21:3693–3698.